Difference Between Probability Density and Radial Distribution Function
Explain the difference between a plot showing the probability density for an orbital and one showing the radial distribution function. At a somewhat superficial level the function ψ is a solution of the Schrödinger equation and effectively describes a wave and it defines an amplitude.
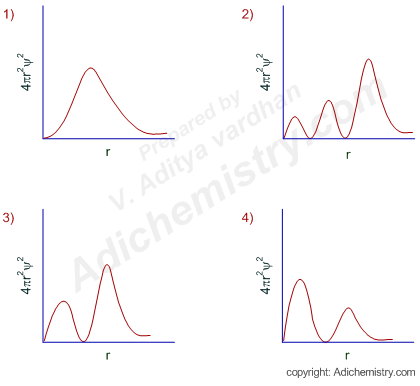
Radial Probability Distribution Curves Atomic Orbitals
Saying that the radial distribution function is zero means that electrons will never be found within that volume.

. The best way to vizualize it is as a group of 3 dimentional projections of normal distributions on a space where each point is the mean value and the function describes the probability density distribution arround each point. For a 1s orbital the radial probability density is maximum at the nucleus while the radial distribution function is zero at the nucleus while the maximum radial distribution function is maximum at a particular distance from the nucleus. The plot showing radial distribution function represents the.
This idea is very common and used frequently in the day to day life when we assess our opportunities transaction and many other things. B v2 is maximum at nucleus but 4tr2v2 is. Radial probability distribution at a given radius is the probability per distance that the event occurs in a infinitesimally thin spherical shell at that radius.
Probability density is probability per volume. I understand that probability of finding electron in Probability density decreases as distance increases. The probability of finding an electron at a point in space is given by ψψ where the.
The radial distribution function is a useful tool to describe the structure of a system particularly of liquids. It indicates a higher probability density for the electron near the nucleus. To understand the difference between probability and probability density consider the difference between mass and density.
If we count the appearance of two molecules at separation r from to we can get the radial distribution function. Radial Probability Radial Probability Density x Volume of spherical shell 4πr 2 drR 2 nl r Radial probability distribution or Radial probability function. There are also many points where the value of radial probability distribution is zero and those points are known as radial nodes.
On the other hand probability density psi2 tells us that there is some finite probability that an electron will be found in that very same region of space. The relation between radial probability and radial probability density is given as. Radial probability distribution at a given radius is the probability per distance that the event occurs in a infinitesimally thin spherical shell at that radius.
Stack Exchange Network Stack Exchange network consists of 179 QA communities including Stack Overflow the largest most trusted online community for developers to learn share their knowledge and build their careers. Answer 1 of 2. The radial distribution function gives the probability of finding a particle in the distance from another particle.
It is also known as radial probability density function it is given by 4πr 2 R 2 nl r. Probability is the likelihood of an event to happen. Radial probability distribution at a given radius is the probability density of an electron in an infinitesimally thin spherical shell at that radius and is a function of radial distance from the nucleus.
What is the main difference between the radial distribution function and the radial probability density for the 1s orbital. Where average is given by langle f ranglefracintb_af mathrmdxb-a I hope you have understood the difference between function density and the function itself. It is important to say that probability distribution function is a probability Ie its value is a number between 0 and one and it is defined for both discrete and continuous random variables.
By seeing the graphs you can to a certain extent predict the average of the probability density. The probability density function of a continuous random variable is the derivative of the cumulative distribution function as long as the derivative exists. Probability Distribution Function vs Probability Density Function.
Probability density at a given point means probability per volume in the limit that the volume is infinitesimally small. 4πr2 the radial probability distribution probability per radius. Difference between Probability density and radial distribution.
The plot showing probability density plotted against radius indicates the probability of finding an electron at a point in space. In statistical mechanics the radial distribution function g displaystyle g in a system of particles describes how density varies as a function of distance from a reference particle. And radial distribution increases to a max and then decreases.
In the graphs shown in question ψ 2 is shown. Click hereto get an answer to your question - Which of the following statement concerning probability density v2 and radial distribution function 4tr2y2 for a s-orbital of H-like species is correct. Problem 31 Easy Difficulty.
A Probability density as a function of r is probability of finding the electron at a specific point in space at a distance r from the nucleus whereas the radial probability function of r is Pr is the probability of finding electron at any point at a distance r from the nucleus. Probability density at a given point means probability per volume in the limit that the volume is infinitesimally small. For a 1s orbital the radial probability density is maximum at the nucleus while the radial distribution function is zero at the nucleus while the maximum radial distribution function is maximum at a particular distance from the nucleus.
The radial probability and probability density are something similar. So the maximum we get in Bohr radius is the probability density and not the probability right. A v2 is minimum at nucleus but 4tr2y2 is maximum at nucleus.
The probability density function for the double. So my question is where is the maximum probability of finding an electronIs it at the nucleus or where the radial distribution function. Whats the difference between probability density function and probability distribution function.
If a given particle is taken to be at the origin O and if ρ N V displaystyle rho NV is the average number density of particles then the local time-averaged density at a distance r displaystyle.

Radial Extent Of 4f And 5f Valence Electrons A The Radial Probability Download Scientific Diagram

Computational Chemistry How To Obtain The Radial Probability Distribution Function From A Quantum Chemical Calculation Chemistry Stack Exchange

Quantum Chemistry Identifying Radial Probability Distribution Curve Given N And L Value Chemistry Stack Exchange
No comments for "Difference Between Probability Density and Radial Distribution Function"
Post a Comment